Introduction:
Von Willebrand factor (VWF) carries out its hemostatic roles by interacting with both platelets and FVIII, and its appropriate active multimeric conformation is strongly affected by blood flow shear. Our aim was to assess the hemostatic status and the response to treatments in VW disease (VWD) using a flow chamber-based thrombus formation analysis system (T-TAS ®, Zacros) that allows evaluation of both primary and secondary hemostasis in a scenario closer to in vivo conditions.
Methods:
Blood samples from patients diagnosed with VWD type 1 (n=3), type 2A (n=2), type 2B (n=1) and type 3 (n=8, two of them with inhibitory antibodies) were collected before and after in vivo administration of the prescribed treatment with desmopressin (DDAVP ®) or plasma-derived (pd)VWF/FVIII concentrates (Table-1). The effect of ex vivo administration of 100, 150 and 300 IU/dL of pdVWF/FVIII (Fanhdi ®, Grifols) was also evaluated.
Thrombus formation analysis was performed according to the manufacturer's standardized protocols: Blood samples collected in tubes containing BAPA (inhibitor of FXa and thrombin) were loaded onto type-1 collagen-coated platelet (PL)-chips to assess platelet-dependent thrombus formation. Citrated blood samples were recalcified in presence of CTI and loaded onto collagen/tissue factor-coated atherome (AR)-chips to assess thrombus formation mediated by both platelet and coagulation activation. An optimized flow rate is automatically adjusted for each chip. Area under the flow-pressure curve (AUC) values were collected.
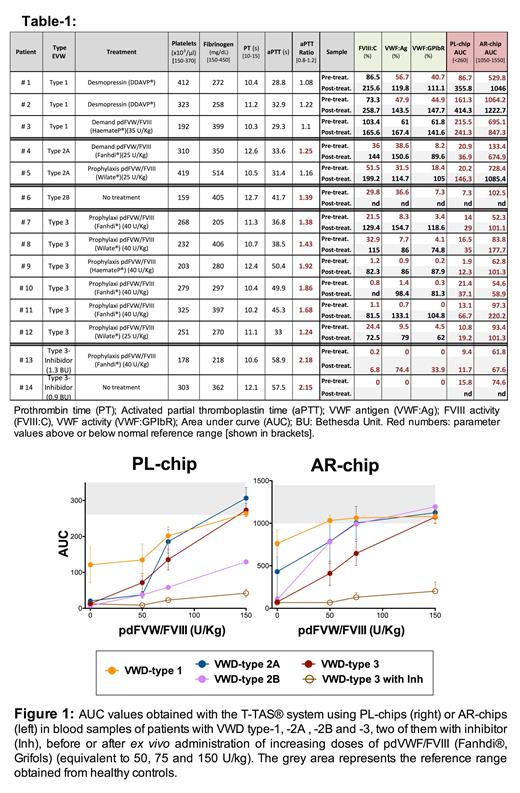
Platelet count, fibrinogen levels, prothrombin time (PT), activated partial thromboplastin time (aPTT), aPTT ratio, antigenic VWF (VWF:Ag), VWF activity by recombinant GPIb binding assay (VWF:GPIbR) and FVIII activity (FVIII:C) were also analyzed.
Results:
All VWD patient samples tested, including type-1 patients, showed a deficient platelet function (PL_AUC) and a reduced coagulation-dependent thrombus formation (AR_AUC) prior to treatment (Table-1). VWD type-1 responded to desmopressin with a high increase of FVIII activity (FVIII:C >200%) and normalization of VWF activity (VWF:GPIbR >100%) and AUC levels. Treatment with pdVWF/FVIII increased VWF:GPIbR levels (>60% after treatment) in all cases, but failed to completely normalize the AUC values, which showed a slight increase (Table-1). A high correlation (r>0.8) was observed between plasma levels of VWF or FVIII activity and AUC values obtained both before and after treatment
Ex vivo administration of pdVWF/FVIIIa resulted in a concentration-dependent increased of PL_ and AR_AUC, with values within the normal range at the highest dose tested (300 IU/dL, equivalent to 150 IU/kg) in all cases, except for VWD type-3 samples with inhibitor and for VWD type-2B sample with PL-chip assay (Figure-1).
Conclusions:
Monitoring treatment efficacy in VWD using a flow chamber-based thrombus formation analysis system could be a reliable and standardized method to assess the response to desmopressin and pdVWF/FVIII concentrates in patients with VWD, allowing an optimal personalized therapeutic dosing and a better prevention of bleeding episodes in these patients.
Funding: Grifols; Zacros; Fundación Rodríguez Pascual; Instituto de Salud Carlos III (ISCIII) (PI22/01461), co-funded by the European Union.
Disclosures
G Arias-Salgado:Grifols: Research Funding; Novo Nordisck, Novartis: Speakers Bureau. Martin Salces:Novo Nordisck, Novartis: Speakers Bureau. Rivas Pollmar:Novo Nordisck, Novartis: Speakers Bureau. Butta:Takeda, Novo Nordisck: Research Funding; Novo Nordisck, Novartis: Speakers Bureau. Hermans:Bayer, Takeda, Roche, CSL Behring, Novo Nordisk, Pfizer, Sobi, LFB, OctaPharma, Uniqure and Biomarin: Consultancy. Jiménez-Yuste:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Álvarez-Roman:Grifols: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; LFB: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau.